The assessment of the effect of carbon dioxide on the water-chemical modes of steam boilers
Ivan Tikhonov
In the design and operation of low-pressure steam boilers there is a number of significant difficulties in the organization of water-chemical mode (WCM) of their work.
Water-chemical mode (WCM) of operation of a steam boiler should ensure trouble-free and cost-effective operation of the equipment and pipelines of the boiler.
WCM of a boiler is to maintain a certain composition of feed, make-up, boiler water, steam and condensate, which does not cause damage to the boiler equipment or its inefficient operation.
Water with dissolved salts and gases is a stable thermodynamic system in equilibrium with external conditions. When external conditions change, such as an increase of temperature of water, the system tries to achieve a new equilibrium state. In this case, it is possible that there is the precipitation of salts in the form of a solid substance from water or, conversely, an increase in solubility for any substance, or the release of dissolved gases, etc.
Thus, in order to ensure the effective management of the steam boiler house, the initial water must be prepared in such a way that the composition of the boiler water and condensate obtained in the process of operation does not cause damage to the equipment.
Until the early 2000s, the main technology of water treatment for low-pressure steam boilers was deep Na–softening of the source water. Indeed, this technology is quite easy to operate, it has a low cost of the used equipment. The quality of the prepared water largely ensures trouble-free operation of the steam boiler and other equipment. The main disadvantages of this technology include a large number of highly mineralized wastewater which is generated during the regeneration of the softening unit, as well as the fact that this technology does not reduce the total salt amount of water, but even increases it slightly.
The first drawback is almost impossible to cope with. The amount of salt which is discharged from the softening unit is equal to the amount of salt which is absorbed on the softening unit multiplied by about 2. This colossal amount of salt should be dissolved in water with a concentration of not more than 1000 mg/l when it is discharged into the sewer. Thus, when the salt amount of initial water is about 250 mg/l, the amount of waste water with salt concentration of 1000 mg/l will be2 times less than the amount of softened water per filter cycle.
Evaporation is another way to recycle Na – softening effluents. Evaporation is also a quite expensive and difficult technology. This eliminates the usage this way, especially for boilers of small power.
However Na-softening of water, virtually not having alternative technology, has been used everywhere for more than 100 years. For the last 15-20 years, the technology of reverse osmosis desalination of water has been actively used in heat power engineering. The main drawback of this technology is the high cost of the equipment used and, as it is believed, a large amount of generated effluents. Although the amount of salt discharged with these effluents is about two times less than the amount of salt formed during the operation of Na – softening. This circumstance requires careful consideration and this will be the subject of other article.
The principal difference between reverse osmosis technology and softening is that reverse osmosis removes all suspended and organic substances from the water, as well as dissolved substances in an amount of about 96-99%.
Thus, reverse osmosis significantly (almost completely) reduces the amount of dissolved substance (all types of ions) contained in the source water, while Na – softening only replaces calcium and magnesium ions with sodium.
In the classical understanding of the water-chemical mode of steam boilers, the value of the salt amount of feed water affects only the amount of continuous blowdown of the boiler. Continuous blowdown of the steam boiler is calculated by the formula:
 , % (1)
, % (1)
Qmw – the share of make-up water;
Stw – the salt amount of treated water, mg/kg;
Sbw – the salt amount of boiler water is taken according to the passport data of the boiler manufacturer, mg/kg.
The greater the proportion of condensate return, the smaller the percentage of continuous blowdown of the boiler is. The value of continuous blowdown of steam boilers up to 10% of steam capacity is allowed. The decrease of the continuous blowdown of the boiler adds significantly to the saving of fuel gas. Thus, the greater the value of the return of desalted condensate to the boiler, the smaller the value of continuous blowdown is. At value of continuous blowdown less than 1 % it is not made, and the implementation of standard of salt amount of boiler water is made only by means of periodic blowdown. At that there is a significant saving of fuel gas.
In the absence of a condensate return to the boiler or with the slight return of the condensate, the use of reverse osmotic desalination of make-up water also eliminates the need for continuous blowdown of the boiler.
If there is some clarity about the effect of the mineral composition of the water, then there is some misunderstanding about the effect of carbon dioxide (CO2) on WCM of the steam boilers. And as practice shows, influence of CO2 dissolved in the source water on the entire WCM of boiler house is often not taken into account in the design and operation of steam boilers.
In this article an attempt is made to describe the effect of carbon dioxide dissolved in the source water on WCM of the boiler house, and recommendations are given to reduce the negative impact of carbon dioxide on the equipment and pipelines of the boiler house.
When steam boilers are operated with the wrong WCM equipment, relating to the steam-condensate path (steam traps, pressure regulators, etc.), fails most often. The steel economizer of the steam boiler also most often fails.
Even if the boiler remains in operation when this equipment fails, it entails a significant reduction in the economic efficiency of the boiler operation.
For example, the installation of expensive steam traps with a long service life fully pays for itself with a high-quality process of condensate drainage and a long service life. But when WCM of boiler is incorrect, as practice shows, even the most expensive and high-quality steam traps and other equipment of the steam condensate path fails in just six months.
Water can exhibit both corrosion and scale-forming properties with respect for the contact surface. Hardness ions are involved in the process of scale formation and are removed at the stage of water treatment.
Elements of the heat scheme, where the water is at high pressure and temperature are: the line of make-up water with a heat exchanger in front of the deaerator; deaerator; feed line with the pump; economizer; steam boiler; steam condensate path with equipment.
In these elements of the thermal scheme of the boiler scale formation and corrosion can occur. In the absence of hardness ions in the water the main problem in the operation of the boiler becomes corrosion.
This type of corrosion is electrochemical corrosion and occurs with oxygen and hydrogen depolarization. Simply put, this corrosion occurs in the presence of oxygen and hydrogen ions in the water. Oxygen exists in make-up water and its amount for “clean” water depends on the water temperature. At zero degrees, the water contains 14 mg/l O2. This is true for water that has a sufficiently long contact with the atmosphere and is in equilibrium with it in oxygen. Artesian water, which has no long-term contact with the atmosphere, and is not aerated, may contain much less oxygen.
Thus, make-up water always contains enough oxygen for corrosion processes. Oxygen corrosion is extremely fast when water boils or steam condensates.
Corrosion with hydrogen depolarization occurs in the presence of hydrogen ion in water, in other words, acid. When carbon dioxide is dissolved in water, H2CO3 carbonic acid is formed, which dissociates into hydrogen ions H+ and HCO3– bicarbonate. Hydrogen ion causes active corrosion processes.
It should be noted that the mobility of hydrogen ions and oxygen molecules in water differs greatly. The hydrogen ion is much more mobile. Therefore, acid corrosion is much faster than oxygen corrosion and is virtually independent of hydrodynamic conditions. It must be said that if there are conditions for corrosion with oxygen and hydrogen depolarization at the same time, the total corrosion rate is much greater than for the corrosion only with oxygen or hydrogen.
It is noted that the water in the state of saturation (boiling) shows active corrosion with oxygen depolarization, as boiling water contributes to a significant increase in oxygen mobility. Hydrogen corrosion on the contrary decreases with increasing water temperature. This circumstance is typical for water of condensate type. The author knows the case when the condensate return pipeline with a temperature of 40 0C required a complete replacement once every six months. In this case, the pipelines of the returned condensate with a temperature of 100 0C and more did not require such frequent repairs, although the iron amount in the condensate was more than 1 mg/l (quite significant). This is probably due to the fact that carbonic acid is gradually converted into carbon dioxide gas at the increase of water temperature. At this, the greatest effect of the release of carbon dioxide from the water occurs when the water boils.
Two technologies are currently used to remove dissolved gases from water; Thermal degassing of water and chemical deaeration with alkalization of the water.
Strictly speaking, removal of gases from water occurs only at thermal degassing. Dissolved gases only bind and remain in water during chemical deaeration and water alkalinization.
The binding of oxygen occurs by reactions:
When using sodium sulfate
2Na2SO3+O2–>2Na2SO4 (2)
When using hydrazine hydrate
N2H4*H2O+O2–>3H2O+N2 (3)
The turning of carbon dioxide into bicarbonate ion (alkalization) occurs by the reaction:
NaOH+H2СO3=NaHCO3+H2O (4)
Chemical deaeration and alkalinization has a number of significant disadvantages:
- The salinity of the feed water significantly (for surface water up to 50 percent or more) increases when chemical deaeration has been carried out and, respectively, the continuous blowdown of steam boiler increases. To bind 1 mg of oxygen, 10 mg of sodium sulfite is spent. It should be noted that when using hydrazine hydrate, the salt content of water does not increase, but the reagent itself is extremely toxic (belongs to the first class of danger), fire hazardous and requires specific storage conditions, which excludes its use for steam boilers, especially those working in food production.
- Sulfites remain in the water after the chemical deaeration, this is due to their excessive dosing for the reliable bonding of oxygen. As a rule, boiler manufacturers strictly regulate the content of sulfites in boiler water (5-10 mg/l), which presents a significant difficulty in the organization of the process of dosing of sodium sulfite into feed water. Sulfite ion (SO3) is a strong reducing agent and significantly enhances the corrosion processes occurring in the boiler and the steam condensate path by destroying the passivating layer on the metal surface. Interaction of sulfite ion with the product is not allowed. Sodium sulfite belongs to the substances of the 3rd hazard class. Sodium sulfite is most suitable for binding a small amount of residual oxygen in the feed water after thermal deaerator.
- Another point that is not always taken into account is that when sodium sulfite is dosed into water, sodium sulfate Na2SO4 is formed, which, in fact, increases the content of ion sulfate in the feed water and when hardness salts enter the boiler insoluble calcium sulfate CaSO4 (gypsum) may be formed in the boiler, it is possible to form insoluble calcium sulfate CaSO4 (gypsum). Calcium sulfate forms dense deposits (contamination) on the evaporative surfaces, which significantly increase the thermal resistance, and leads to overheating of the pipe metal and significant waste of flue gas. Moreover, gypsum is almost impossible to remove from the surface of the pipes by chemical washing of the boiler with inhibited hydrochloric acid.
- Increasing the alkalinity of the feed water with caustic soda only turns carbon dioxide into sodium bicarbonate (equation 4), which will again pass into carbonic acid in the boiler, it will be released as carbon dioxide into the steam when the water boils and subsequently passes into the condensate, causing a decrease in the pH of the condensate and significantly increasing its corrosion properties. Thus, the alkalinization of feed water helps to avoid only carbon dioxide corrosion of the feed and boiler path of the steam boiler, but increases the corrosion aggressiveness of the condensate.
Thermal degassing of water allows not only removing oxygen and carbon dioxide from the water, but also significantly reduces the corrosion properties of the returned condensate.
Let’s make the material balance of carbon dioxide for the thermal scheme of the boiler in Fig.1,2
Before drawing up the balance of carbon dioxide we should thoroughly understand the concepts of the so-called free, bound and semi-bound carbon dioxide contained in water.
Let’s do a simple mental experiment. There is a certain amount of water. The pure water contains only molecules of H2O. The pH of water is 7.0. That is, the number of hydrogen ions is equal to the number of hydroxyl ions. Suppose that this water is in contact with solid calcium carbonate. Calcium carbonate is soluble only in water having an acid reaction. In other words, the water has a greater number of hydrogen ions in relation to the hydroxyl ions. Hydrogen ions can be introduced into water either with strong acids such as hydrochloric acid or with weak acids such as carbonic acid. There is a fundamental difference between strong and weak acids. So in strong acids, the hydrogen ion responsible for the acid reaction has been introduced or already exists in water with its own anion (Cl, SO2). A weak carbonic acid dissolved in water is formed from carbon dioxide and water by binding the hydroxyl ion of the water molecule into the bicarbonate ion, and accordingly the acidic residue remains as hydrogen.
The equation of the reaction of dissolution of solid calcium carbonate (CaCO3) in water with carbonic acid which enters into the water from the air, from various processes of oxidation of organic carbon, or from geological processes:
CaCO3+H2CO3<-->Ca(HCO3)2 (5)
The reversible reaction takes place, in which the solid calcium carbonate (CaCO3) passes into the dissolved form of calcium bicarbonate (Ca(HCO3)2). Carbon dioxide in the form of carbonate, which is associated with calcium, is the binding form of carbon dioxide, which is equal to the half of the calcium of bicarbonate dissolved in water. The second half of calcium bicarbonate, which is not formed from solid calcium bicarbonate, but from carbonic acid, is called semi-bound carbon dioxide.
Free carbon dioxide in water is in the form of carbonic acid (H2CO3), i.e. it is not directly related to any cation in water. However, free carbonic acid, which is part of the water’s carbon dioxide buffer system, determines the current pH value of the water.
Let’s go back to our experiment. It turns out that free carbon dioxide (H2CO3) is formed in the water by passing carbon dioxide (CO2) through our water volume. Free сarbon dioxide reacting with calcium carbonate (limestone) by equation (5) dissolves it to form calcium bicarbonate. Moreover, half of the bicarbonate ion, measured in molar concentrations, belongs to the bound form of carbon dioxide and the other half to the semi-bound (coming from carbon dioxide). Dissolution of limestone will take place as long as carbon dioxide is passed through the water. In this case, the increase in the concentration of bicarbonate ion in the solution will require an increase in the concentration of carbon dioxide in the water to maintain the dissolution process.
The system will begin to come to a new equilibrium state after the end of passing carbon dioxide through the water. The dissolution of limestone will end and the gradual release of dissolved free carbon dioxide into the surrounding air will begin. In the earth’s atmosphere, the average concentration of carbon dioxide is about 0.4 mg/l. Accordingly, under ideal conditions, the concentration of carbon dioxide in water should reach the same concentration. But in nature, the concentration of free carbon dioxide in open flowing waters is from 2 to 5 mg / l, which corresponds to the pH value of 7.7-7.9. This is probably due to certain biological and geological processes ensuring the flow of carbon dioxide into the water but not from the atmosphere.
Thus, when carbon dioxide is released from water into the atmosphere, the reaction according to equation (5) proceeds towards the formation of solid calcium carbonate. In the end, the “final” balance is achieved and the water begins to have an ionic composition peculiar to the geological and biological conditions of the area. In our experiment it contacts with calcium carbonate and atmospheric air.
Keeping in mind that there is bound, semi-bound and free carbon dioxide in water, we consider the equation of reactions occurring during thermal deaeration of Na-softened water:
Stage 1
Removal of free CO2 due to boiling water and subsequent removal of CO2 with steam. This process must take place (start and end) in the deaeration column. Water should boil on the column plates. For this, the heating of make-up water before the deaeration column must be provided. The higher the heating to the make-up water is the earlier the boiling in the deaeration column begins and the more efficient and complete the removal of free carbon dioxide from the make-up water is carried out. In the absence of boiling the make-up water in the deaeration column free carbon dioxide can get into the deaerator tank. The CO2 in the tank will be bound into sodium bicarbonate by the sodium carbonate which has been formed there during the decomposition of the previous sodium bicarbonate (according to equation 6 process from right to left) and accordingly, the process of isolation of semi-bound carbon dioxide is significantly slowed down. It will require a much longer stay of water in the deaerator tank and for boiling of water it will require much more steam.
Stage 2
In the second stage, the destruction of the bicarbonate ion occurs due to the continued intensive removal of carbon dioxide from the water to form carbonate (6). This process occurs, as a rule, in a deaerator tank with intensive steam bubbling of water.
2NaHCO3<->Na2CO3+Н2CO3free<-> Na2CO3+CO2gas+ Н2О (6)
The completeness of this process in the deaerator can be estimated by the pH value of the water after the deaerator. The pH value of the water after the deaerator will be determined by the ratio of the carbonate ion to the bicarbonate ion (Henderson-Hasselbalh equation for the decomposition of bicarbonate HCO3<->H+CO3)
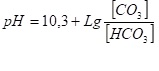 (7)
(7)
For example, water containing 2 moles of sodium bicarbonate (6) is boiling in a deaerator.
As a result of the removal of CO2 bicarbonate ion becomes carbonate ion. Depending on the intensity of CO2 removal a balance between carbonate and bicarbonate is stated in the water at the outlet of the deaerator, while only half of the decomposition of bicarbonate can occurs. Accordingly, as a result of steam bubbling of water, we obtain the amount of 1 mole of sodium bicarbonate, 0.5 mol of sodium carbonate and 0.5 mol of carbon dioxide. Carbon dioxide is diverted to the atmosphere.
Then, according to (7) we obtain:
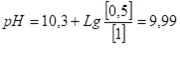
The pH value of the degassed water after the deaerator is 9.99. In this case, all free carbon dioxide and half of semi-bound carbon dioxide (one quarter of the original bicarbonate) was removed in the deaerator.
Suppose ¾ of sodium bicarbonate is destroyed in the deaerator and not half. Then, as a result of the removal of carbon dioxide, one quarter of bicarbonate or 0.5 mol of bicarbonate remains in water and, accordingly, we get 0.5 mol of carbonate 0.5 mol of hydrate and 1 mol of carbon dioxide (which was removed in the deaerator). In this case, exactly half of the carbon dioxide was removed from the initial amount of bicarbonate in the deaerator. In this example, hydrate appears in water as a result of the hydrolysis of carbonates. In this case, equation (7) is written as following:
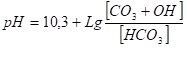
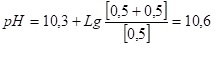
The process of hydrolysis of bicarbonates and carbonates is considered in detail in the article of I. Tikhonov «The method for the determination of the amount of bicarbonates, carbonates and hydrates in water depending on the pH of the water» tiwater.info.
The calculated pH value of the water to be obtained at the outlet of the deaerator corresponds to equal amounts of bicarbonate and carbonate, i.e. bicarbonate, carbonate and carbonic acid will be equal to the amount of 0.665 mol. 0.665*3= 2 mole of the original bicarbonate. Accordingly,
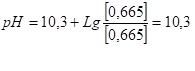
PH=10.3 corresponds to the dissociation constant of bicarbonate according to the equation HCO3<->H+CO3. That is, the amount of bicarbonate is equal to the amount of carbonate. It can be said that at this pH of the water half of the bicarbonate of make-up water is decomposed in the deaerator, but only a third of the sum of all forms of carbon dioxide is removed (8).
0,665NaHCO3<-> 0,665Na2CO3+ 0,665CO2gas+Н2О (8)
Stage 3
The third stage is the continuation of removal of carbon dioxide from water at intensive boiling. At this the carbonate is hydrolyzed to form carbon dioxide and release hydroxyl ion from the water molecule (9). This process does not require removal of carbon dioxide from the water. This is a common reaction of hydrolysis of soda ash in water. Therefore, it is probably wrong to talk about the destruction of bound carbon dioxide (carbonate) due to the removal of gaseous carbon dioxide. It is more correct to talk about the hydrolysis of soda ash in water. These conditions are provided both in the deaerator and especially in the boiler. It can be said that the rate of reaction (9) is limited by the residual amount of bicarbonate in the water, and not by the rate of removal of carbon dioxide. And the concentration of bicarbonate depends on the efficiency of removal of dissolved carbon dioxide (6).
Na2CO3+ H2O<->2NaOH+ CO2gas (9)
Na2CO3+ H2O<-> NaOH+ NaHCO3 (10)
It is believed that the higher the temperature and pressure in the boiler, the more complete the decomposition of carbonates occurs in the boiler water, due to the conditions providing extremely efficient removal of carbon dioxide from the water. Complete decomposition of carbonates occurs in boilers at the pressure of 5 MPa [1]. Nevertheless, the author believes that there is no destruction of carbonates as such. There is their hydrolysis (9, 10). Accordingly, the less bicarbonate in water is, the more complete the hydrolysis of carbonates occurs, and this determines the completeness of the decomposition of carbonate. Thus, when the steam boiler is operated at the pressure of 6 bar and the boiler is fed with demineralized water after reverse osmosis with an alkalinity of about 0.25 mg-eq/l, bicarbonate and carbonate ion are not actually detected in the boiler water. There is only hydroxyl in boiler water (only phenolphthalein alkalinity is present).
Note that after the deaerator, during its normal operation, the water has a pH value, usually about 8.5-10.0, which indicates the simultaneous occurrence of reactions (6) and (9, 10) in the deaerator.
We can write
2NaHCO3<->Na2CO3+Н2CO3semi-bound<-> Na2CO3+CO2gas+Н2О<->2NaOH+CO2gas (11)
As a result, the following processes occur in the deaerator and deaerator column. Make-up water entering the deaerator, as a rule, contains free carbonic acid which does not allow the reaction (6) to be carried out. Bicarbonate begins to pass into carbonate with the gradual removal of free carbon dioxide from the water. In this case, the carbonate hydrolyzed in water into sodium hydroxide and carbon dioxide, which is removed with steam. Sodium hydroxide increases the pH of water.
So, directly all the free carbon dioxide and part of semi-bound carbon dioxide should be separated in the deaerator. As shown above, at a pH value of deaerated water about 10, only half of the semi-bound carbon dioxide is released in the deaerator. Accordingly, the remaining part will go to the boiler, there carbon dioxide will be released into steam and reactions (6) and (9, 10) will proceed simultaneously. In the boiler, the transition of bicarbonate ion to carbonate (6) is almost complete and, accordingly, sodium hydroxide will be formed from carbonate (9, 10). The completeness of the decomposition of the bicarbonates, and not as they say of carbonates, in the boiler will be determined by the amount of alkalinity in the make-up water, the percentage of condensate return, the pressure in the boiler.
If we assume the conditions: all the free carbon dioxide is removed in the deaerator column; Then in the deaerator tank, carbon dioxide is released from the destruction of half of the semi-bound bicarbonate; Then in the boiler, the second half of the semi-bound bicarbonate is released, as well as in the deaerator and in the boiler, hydrolysis of sodium carbonate will occur; Then it can be said that the amount of carbon dioxide equals to a quarter of the amount of bicarbonate ion (alkalinity) of make-up water and it will be removed in the deaerator, and the amount of carbon dioxide numerically equals to three-quarters of the value of the alkalinity of the make-up water and it will be removed in the boiler. The pH value of deaerated water will be 9.99.
We can write down:
СО2fw=3/4*Amw , mmol/l (9)
where,
СО2fw – the concentration of bound and semi-bound carbon dioxide in feed water;
Amw – the alkalinity of the make-up water, mmol/l.
Let’s write the equation of material balance of carbon dioxide for the deaerator (Fig.1).
(1-q)*CO2fw+q*СО2mw=1*СО2fw (12)
where,
q – the fraction of feed water or the fraction of losses of a condensate;
СО2fw– the carbon dioxide concentration in feed water which is the same СО2steam.
СО2mw– the carbon dioxide concentration in make-up water.
This record of the equation of material balance of carbon dioxide in the deaerator assumes that the concentration of carbon dioxide in steam is equal to the concentration of carbon dioxide in feed water. That is, bicarbonates and carbonates are completely decomposed in the boiler, and the condensate is returned to the deaerator tank (not the deaerator column) (Fig.1)
Having solved the equation (12), we obtain
СО2fw=СО2mw=3/4*Amw (13)
It turns out that in the case when the condensate is returned to the deaerator tank without removing the free carbon dioxide, the amount of carbon dioxide in the feed water does not depend on the proportion of condensate return. In the experience of the author, such cases are quite common. Even if 90% of the condensate is returned to the boiler room, if the deaerator is not working properly, the condensate contains a large amount of carbon dioxide and is corrosive.
That is, if the alkalinity of the make-up water is 2.5 mmol/l and the share of make-up water is only 10%, the amount of carbon dioxide in the condensate will be ¾ *2.5=0.93 mmol/l or 0.93*44=41mg/l, which exceeds the allowable amount of carbon dioxide in the steam (20 mg/l) by 2 times. This suggests how important it is to remove carbon dioxide from the condensate when returning it to the deaerator.
When the condensate is returned to the deaerator column, carbon dioxide is removed from the condensate (Fig.2). Then
(1-q)*CO2cond+q*СО2mw=1*СО2fw
CO2cond=0
СО2fw= q*СО2mw (14)
Then for the above conditions
СО2fw=0,1*3/4*2,5=0,093 mmol/l or 4,1 mg/l.
Let us consider the following example:
for the value of the alkalinity of make-up water 2.5 mmol/l, in the case of “ideal” operation of the deaerator, i.e. significant heating of the source water 80C or more; the use of steam on the “mirror” blowing deaeration column; the use of steam for the bubbling, the water temperature in the tank is better to maintain at least 108 C; the time of the water in the deaeration tank should be at least 1 hour. In this mode of operation of the deaerator, we can get the pH value of deaerated water about 10.3 (from my own experience).
Earlier it was shown that 1/3 of carbon dioxide is removed in the deaerator. Accordingly, the concentration of carbon dioxide in the feed water and, accordingly, in the steam will be:
- In the absence of condensate return
СО2fw=1/3*2,5=0,83 mmol/л или 36,7 mg/l.
The concentration of CO2 in the steam is close to the standard (up to 20 mg/l)
- When condensate is returned 50%
СО2fw=1/3*0,5*2,5=0,417 mmol/l или 18,3 mg/l.
- At any percentages of condensate return without removal of carbon dioxide in the deaerator column
СО2fw=1/3*2,5=0,83 mmol/l или 36,7 mg/l.
Even with the “ideal” operation of the deaerator, the return of condensate without preliminary removal of carbon dioxide in the deaerator column leads to an increased amount of carbon dioxide in the steam.
The above examples and calculations assume complete decomposition of bicarbonates and hydrolysis of carbonates in the boiler. However, it is believed that under real conditions, complete decomposition of bicarbonates does not occur. The estimate of the decomposition of bicarbonate and hydrolysis of the carbonate on a working boiler can be carried out on the basis of the analysis for the alkalinity of the boiler water at the phenolphthalein and methylorange. The proportion of remaining carbonate and bicarbonate in the boiler water is determined by the proportion of methylorange alkalinity in the total alkalinity of the boiler water.
∂k =Am.o/(Ap/p+ Am.o) (15)
where,
∂k – The proportion of carbon dioxide remaining in the form of bicarbonate and carbonate in the boiler water after removal of carbon dioxide;
Am.o– Alkalinity at methylorange, mol/l;
Ap/p– Alkalinity by phenolphthalein, mol/l.
For clarification, let’s look at a few examples.
Suppose the alkalinity of the source water is HCO3 = 10 mmol/l. The part of the carbon dioxide is removed from the water by boiling. Then, we analyze the water for alkalinity at(by) phenolphthalein and methylorange. As a result, we obtain that the amount of acid on the titration at phenolphthalein is 1 ml, at methylorange 9 ml.
Let’s write down the decomposition scheme of bicarbonate (The process of decomposition of bicarbonate is described in I. Tikhonov’s article «The method for the determination of the amount of bicarbonates, carbonates and hydrates in water depending on the pH of the water», tiwater.info ):
8,0NaHCO3<-> 1,0Na2CO3+ 1,0CO2gas+Н2О
That is, the concentration of bicarbonate – 8,0 mmol/l, carbonate – 1 mmol/l. The amount of carbon dioxide released is 1,0 mmol/l.
Then,
∂k =Am.о/(Ap/p+ Am.о) =9/(9+1)=0.9
0.9 – The proportion of carbon dioxide remaining in the form of bicarbonate and carbonate in water.
That is, only 10% of the carbon dioxide has been removed, which corresponds to the scheme of decomposition of the bicarbonate. The initial bicarbonate is 10 mmol/l, the amount of removed carbon dioxide is 1.0 mmol/l.
Let’s continue removing carbon dioxide from the water and get the following values:
As a result of the analysis: the amount of acid at phenolphthalein-6 ml, at methylorange-4 ml.
Let’s write down the decomposition scheme of bicarbonate:
2,0NaHCO3<-> 2,0Na2CO3+ 2,0CO2gas+Н2О<->4NaOH+4CO2gas
The concentration of bicarbonate – 2.0 mmol/l, carbonate-2.0 mmol/l, hydrate-4 mmol/l. The amount of carbon dioxide released is 2.0+4.0=6 mmol/l.
Then,
∂k =Am.о/(Ap/p+ Am.о) =4/(6+4)=0,4
0.4 – The proportion of carbon dioxide remaining in the form of bicarbonate and carbonate in water.
That is, only 60 % of carbon dioxide has been removed, which corresponds to the decomposition scheme of bicarbonate. The initial bicarbonate – 10 mmol/l, the amount of removed carbon dioxide – 6.0 mmol/l.
Here is an example from author’s experience: Steam boiler; Steam pressure is 8 bar; Na-softened water is used as a feed water with the alkalinity 2.0 mmol/l; There is no deaerator column; The condensate is returned directly to the deaerator tank in an amount of 70% of the steam capacity; The share of make-up water is 0.3; The temperature in the deaerator tank is maintained at 90-99 C.
The measured alkalinity of boiler water:
Ap/p=20,0 – 21,0 mmol/l
Am.о=1,5 – 2,0 mmol/l
Then
∂k =1,5/(20+1,5)=0,07 or as a percentage – 7 %
That is, the amount of bicarbonate and carbonate remaining in the boiler water is 7% of the amount of bicarbonate and carbonate entering the boiler.
Thus, for this example, we can write that the concentration of carbon dioxide in the steam is:
СО2steam=(1-0,07)* Afw=0,93*2,0=1,86 mmol/l or 81,84 mg/l
Afw – the alkalinity of feed water, mmol/l.
The concentration of carbon dioxide in the steam is much higher than the standard.
If in this example the deaerator column would be installed on the deaerator tank and the carbon dioxide removed from the condensate in the deaerator column, we obtain the following:
СО2steam=0,3*(1-0,07)* Afw=0,3*0,93*2,0=0,558 mmol/l or 24,5 mg/l
It almost meets the requirements of the standard.
As a result, we can write the following formula to calculate the concentration of carbon dioxide in steam, provided that the carbon dioxide is removed from the returned condensate:
СО2steam=44*q*(1-∂k) *Afw= 44*q*(1- ∂k)*∂d*Amw, mg/l (16)
where,
q- the share of make-up water;
Afw– the alkalinity of feed water, mmol/l;
Amw– the alkalinity of make-up water, mmol/l;
Afw=∂d* Amw, mmol/l;
∂d– the share of bicarbonate and carbonate remaining in feed water after their partial removal in the deaerator with the steam. For operating boilers ∂d is calculated by the formula:
∂d= Ad.m.о /(Ad.p/p+ Ad.m.о) (17)
Ad.p/p, Ad.m.о – the alkalinity at phenolphthalein and methylorange of deaerated water, mmol/l.
For preliminary calculations, while providing “ideal” conditions for the operation of deaerator (steam supply to the “mirror” and steam bubbling of tank), it is possible to accept, ∂d = 0.65-0.85. When the deaerator is operated without steam bubbling of deaerator tank, the proportion of bicarbonate and carbonate remaining in the deaerated water is 1 (∂d =1).
∂k – The proportion of bicarbonate and carbonate that are remained in the boiler water. ∂k is calculated by the formula (15). For preliminary calculations ∂k is taken depending on the operating conditions of the boiler, the initial alkalinity and the proportion of condensate return. ∂k=0.05 – 0.2. The greater the alkalinity of the source water the greater the fraction ∂k should be taken.
The formula for calculating the concentration of carbon dioxide in steam when condensate is returned directly to the deaerator tank:
СО2steam=44*(1-∂k) *Afw= 44*(1- ∂k)*∂d*Amw, mg/l (18)
Conclusion:
- Water-chemical mode of steam boilers depends primarily on the finding of three forms of carbon dioxide in water. The ratio of forms of carbon dioxide in water (free carbon dioxide, bicarbonate, carbonate) determines the pH value of water, and, accordingly, its ability to deposit salts or corrosion aggressiveness. It is possible to conduct an effective WCM of low-pressure steam boiler without the use of additional chemicals by correcting the ionic composition of water, as well as the amount of free carbon dioxide in the water.
- Thermal degassing is an effective way to remove corrosive gases from feed water before the steam boiler. Chemical degassing can be used in exceptional cases for small-capacity boilers and with the appropriate economic justification, taking into account the risks of increased corrosion activity of the condensate, the risks of possibility of incomplete binding of oxygen in the feed water, as well as losses with increased consumption of continuous blowdown of the boiler. Also, chemical degassing requires the production of additional tests for the control of WCM. It is possible to use chemical degassing using reverse osmosis membranes (Article: Ivan Tikhonov – Water degassing using reverse osmosis membranes tiwater.info). This technology has no disadvantages that are inherent in conventional chemical degassing.
- Thermal deaeration (degassing) is an important part of the thermal scheme of the boiler. Maintaining the wrong mode of operation of the deaerator entails significant economic losses.
- The most important parameter of the quality of the deaerator is the temperature of the water entering the deaeration. At a water temperature of more than 85 C, the water boils quickly on the upper plate of the deaerator column. This condition determines the completeness of the removal of oxygen and free carbon dioxide from the water. In case of absence of water heating or insufficient heating, the deaerator column in the upper part operates not in the heat – mass transfer mode, but only in the heat transfer mode. As a result, when the height of the column is short or when there is a small supply of steam to the “mirror”, oxygen and free carbon dioxide enter the deaerator tank, while the efficiency of oxygen release decreases significantly, and free carbon dioxide partially binds into bicarbonate. In this case, in order for sodium cationed water to reach a pH value of at least 8.5 a large steam consumption for the bubbling of the deaerator tank will be required. As a result, there are large losses with the evaporation of deaerator.
- Thermal deaeration does not provide the necessary conditions for the removal of bound and semi-bound carbon dioxide from water. Therefore, this stage is wrong to be considered as significantly affecting the value of carbon dioxide concentration in steam and condensate. It is necessary to use water treatment technologies that significantly reduce the amount of alkalinity in the make-up water. This will reduce the concentration of carbon dioxide in a steam and condensate.
- The steam condensate from steam consumers must be returned to the top of the deaerator column to remove free carbon dioxide, which enters the steam as a product of decomposition of bicarbonate in the boiler.
- Knowing the pH value or phenolphthalein and methylorange alkalinity of feed water after the deaerator, we can determine how effectively the deaerator removes carbon dioxide from the water.
- An interesting point, in accordance with the requirements of the standards, the pH value of feed water for low – pressure steam boilers must be in the range of 8.5-10.5. It is considered that with proper operation of the thermal deaerator exactly half the amount of all forms of carbon dioxide in the water should be driven away in the deaerator. That is, the reaction is complete (6). The pH value of such water will be 10.6!
Figure 1

© 2018 Tikhonov Ivan. tiwater.info
Figure 2

© 2018 Tikhonov Ivan. tiwater.info
References
- Водоподготовка и водный режим энергообъектов низкого и среднего давления. Справочник. Ю.М. Кострикин, Н.А. Мещерский, 1990 г.

