Waste water from the Na ion exchange plant. Composition. Disposal method.
Ivan Tikhonov
Na – cation exchange water softening is perhaps the most common process for preparing water for various industrial and drinking purposes. The meaning of this process is that dissolved polyvalent ions are replaced by sodium ions in water. As a result, the water softens and heavy metals are removed from the water.
This process has two significant drawbacks. First, softened water contains only sodium as cations. For production purposes, this is sometimes useful. But this is mainly attributed to disadvantages. And secondly, and most importantly, during the operation of the plant, a large amount of highly mineralized wastewater is generated. This waste water must not be discharged into the sewerage system. Mineralization of these effluents is about 20 g/l (ppt). For discharge into the sewer, water with a mineralization of not more than 1.0 – 1.5 g/l is allowed.
The question arises: what should be done with such effluents, dilute or purify (precipitate)?
In enterprises where water softeners consume very little water in the total balance of water consumption and wastewater disposal, the wastewater from the softener is diluted in a natural way. That is, if the discharge of water from the softening unit into the general sewage collector of the enterprise is, let’s say, 1% of the total wastewater of the enterprise and the rest of the wastewater is low-mineralized, the highly mineralized wastewater of the softener will not significantly affect the total salinity of all wastewater. Thus, in this case, additional treatment of the waste water from the softening unit is not required, since they are naturally diluted with the rest of the enterprise’s effluents.
But what should we do when the remaining effluents of the enterprise are not enough for natural dilution of the effluents from the softening unit or the other effluents are already highly mineralized by themselves?
In this case, either the dilution of effluents with source water or the reduction of salinity of effluents by precipitation of salts from them is required.
Diluting the effluent from a water softener is, logically, a rather strange disposal method. For example, if the salinity of the source water is 0.35 g/l and the filter cycle of the softening unit is 100 m3, then to obtain waste water with salinity up to 1.0 g/l, it will be necessary to consume another 70 m3 of source water with salinity after each filtration cycle during the regeneration process with 0.35 g/l to obtain mixed effluent with a salt concentration of 1.05 g/l. It turns out to be quite unreasonable, a kind of economically completely unacceptable condition. It is required to spend 170 m3 of source water to obtain 100 m3 of softened water.
In such conditions, enterprises naturally simply discharge the waste water from the softener into the sewer without any dilution.
But as it was said, if for enterprises with a small amount of wastewater from the softening system in the general balance, this is quite acceptable, then where softening has a significant share in the total balance of water consumption, it is necessary to apply technologies to reduce the salinity of wastewater by precipitation of salts.
In order to thoroughly understand the process of precipitation of salts of highly mineralized wastewater from a softening plant, it is necessary to clearly understand their chemical composition and the reason why they are.
Figure 1 shows a diagram of a softening plant with the indicated chemical composition of the source water, softened water, regeneration solution and the resulting wastewater. It also indicates which salts can be removed into the sediment and which ones can be returned to the regeneration cycle. In fact, when using this sedimentation technology, only solid sludge of a certain moisture content is formed as effluent.
The source water contains Ca, Mg, Na and anions HCO3, SO4, Cl as cations. As a result of the process of Na ion exchange, Ca and Mg ions are replaced by Na ions. As a result, softened water contains only Na cations, while the anionic composition does not change. After depletion of the cation exchanger in terms of Na ions, the cation exchanger is regenerated by passing through it a solution of sodium chloride (NaCl) with a concentration of 6-8 g/l. As a result, there is a reverse replacement of Na ions contained in the regeneration solution for Ca and Mg ions contained on the cation exchanger. During the regeneration process, wastewater is formed, which contains Ca, Mg and Na as cations, and only chloride (Cl) as anions.
It turns out that the waste water of softening plants contains three salts:
CaCl2, MgCl2, NaCl.
Calcium and magnesium chloride can be precipitated from waste water by adding technical and caustic soda (Na2CO3, NaOH) to it. Thus, if sodium carbonate and sodium hydrate are added to this water, the following chemical reactions will occur:
CaCl2 + Na2CO3 = CaCO3 (precipitate) + 2NaCl (1)
MgCl2 + 2NaOH = Mg (OH) 2 (precipitate) + 2NaCl (2)
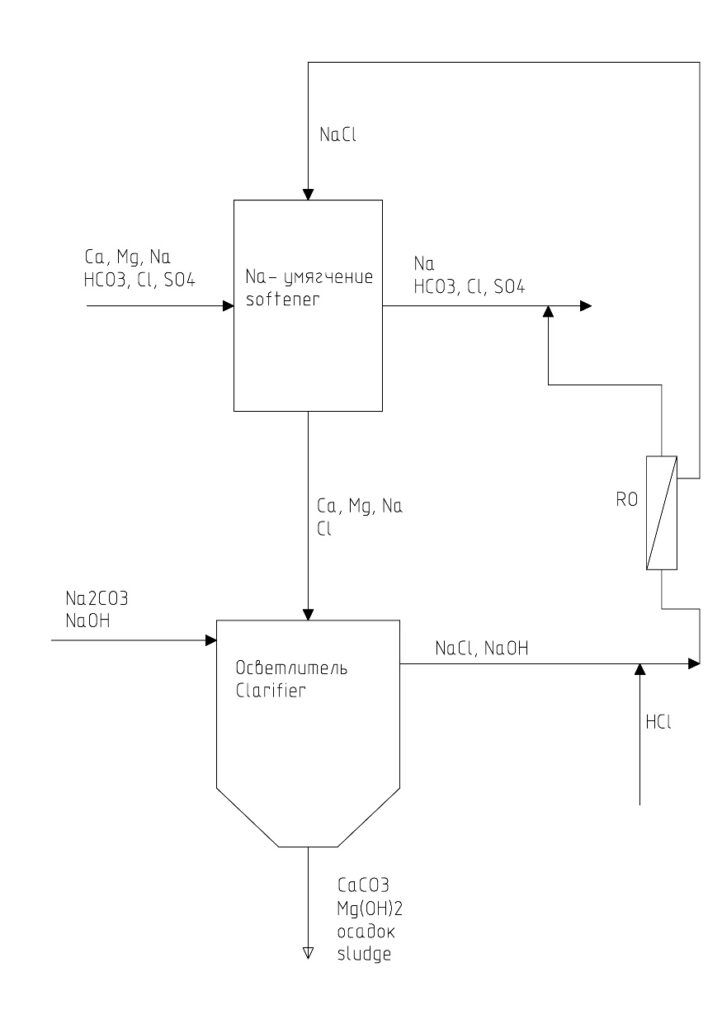
Figure 1
As a result, calcium carbonate and magnesium hydrate salts will be formed in the form of sludge. In return, an equivalent amount of sodium chloride will be generated in the wastewater treated in this way.
Theoretically, the obtained sodium chloride in the treated waste water will be enough to carry out the next regeneration of the water softener. Therefore, the waste water after precipitation of hardness salts from it can be used as a regeneration solution for the cation resin of the softener. This results in a kind of regeneration cycle, from which hardness salts are removed and at the same time an equivalent amount of Na is added. In this case, the anionic composition of water does not change in any way.
In fact, we get a process in which not table salt (NaCl), but caustic and soda (NaOH, Na2CO3) are used as salt for the regeneration of the cation exchanger.
Technical and economic calculations show that the cost of caustic soda and soda for one regeneration is less than the cost of tableted salt. And this is done despite the fact that wastewater is being recycled. Thus, waste water is practically absent and the cost of salt for regeneration is reduced, because its composition is changing.
For a visual representation of this technology for the disposal of wastewater from a softening plant, consider an example.
There is a need to soften water of the following composition:
Ca – 2 mg-eq/l
Mg – 1 mg-eq/l
Na – 1 mg-eq/l
НСО3 – 2 mg-eq/l
SO4 – 0.5 mg-eq/l
Cl – 0.5 mg-eq/l.
The flow for softened water must be at least 5 m3/hour.
Let’s calculate the required diameter of the softening filter.
To do this, it is necessary to find the required filtration area, which is determined by dividing the hourly productivity by the linear filtration rate:
5/25 = 0.2 m2
Where,
25 – recommended linear filtration rate for the first stage of softening, m/h.
Now we determine the diameter of the filter:
D = (0.2 / 0.785) 0.5 = 0.5 m.
Based on the diameter being equal to 0.5 m, we select the filter housing with a standard size 18-65. The real diameter of this body is 0.45 m. The real filtration area is 0.45 * 0.45 * 0.785 = 0.16 m2. The height of the resin layer in the body is 1.1 m. It turns out that the total volume of resin in the body is 0.16 * 1.1 = 176 liters of resin.
The total exchange capacity of the cation exchanger poured into the filter is 176 * 1.2 = 211 g-eq.
Where, 1.2 is the specific exchange capacity of one liter of resin, g-eq/l.
The filter cycle of the water softener is:
211 / (2 + 1) = 70 m3
Let’s calculate the amount of sodium chloride required for the regeneration of the cation exchanger after the completion of the filtration cycle.
176 * 150 = 26 400 g or 26.4 kg of NaCl.
Where, 150 g/l is the specific consumption of sodium chloride for the regeneration of one liter of cation exchanger.
Let’s calculate the amount of required regeneration solution with NaCl concentration being equal to 7.4% or 80 g/l.
26400/80 = 330 liters.
Thus, in order to regenerate the cation exchange resin, 330 liters of a 7.4% solution of sodium chloride are required.
After direct regeneration, it is necessary to wash the cation exchanger from the regeneration products. Source water is used to wash the cation exchanger. Washing one liter of cation exchanger requires about 7 liters of source water.
It turns out that 176 * 7 = 1232 liters of source water is required to wash 176 liters of cation exchanger. It turns out that in total 330 + 1232 = 1562 liters of source water are spent on regeneration. This consumes 26.4 kg of table salt. Accordingly, the average salinity of the received wastewater will be 26400/1562 = 16.7 g/l.
The mineralization of the initial water can be neglected as insignificant in relation to the salinity of the regeneration solution and the wash water.
All waste water must be collected in a tank. Then, sodium hydrate and sodium carbonate are added to the tank in a slight excess of the required equivalent amount. The process of formation of sludge of calcium carbonate and magnesium hydrate begins. After the completion of the reaction, the sludge is removed in the lower part of the tank, and a solution with a concentration of about 17 g/l, but containing only NaCl salts, is taken from the upper part. It is necessary to add hydrochloric acid to this solution to bind sodium hydrate to table salt and reduce the pH of the solution. Then, using known methods (evaporation or reverse osmosis), it is necessary to concentrate this solution to a working concentration of 6-8%. And even at this stage this working solution can be sent to the next regeneration of the cation exchanger.
As you can see, there is no wastewater in this mode of operation of the softener. Tableted table salt is used only during the first regeneration of the cation exchanger. Further, only technical and caustic soda is used for regeneration and at the same time for the disposal of wastewater. That part of table salt (about half of the total amount), which ensures the process of ion exchange of Na from the regeneration working solution for Ca and Mg, remains in the circuit all the time and is not removed anywhere. It enters the regeneration circuit during the first regeneration. The amount of Na substituted on the resin for hardness ions enters the regeneration circuit with technical and caustic soda, while ensuring the process of sludge formation from hardness salts.
We can say that the total amount of technical and caustic soda will be approximately two times less than the amount of tableted salt required for the same regeneration. Therefore, at approximately the same cost of soda and tableted salt, the total costs for regeneration will be two times lower when using soda under conditions of precipitation of hardness salts.
Let us carry out an approximate technical and economic calculation of the cost of operating a Na unit – water cationization with a system for utilizing the generated wastewater from the cation exchanger regeneration process.
To begin with, using the data of the above presented example, we will calculate the required amount of technical and caustic soda for the regeneration of the cation exchanger and, correspondingly, for the precipitation of hardness salts.
Let us calculate the amount of Ca and Mg cations formed on the cation exchanger in one filtration cycle.
Calcium amount:
The proportion of calcium in the total hardness – 2 / (2 + 1) = 0.67
Then, 211 * 0.67 = 141 g-eq.
Magnesium amount:
The proportion of magnesium – 1 / (2 + 1) = 0.33
Then, 211 * 0.33 = 70 g-eq.
According to the reaction equation (1), 1 mol of sodium carbonate is required to precipitate 1 mol of calcium chloride.
We get that 141 g-eq of sodium carbonate is required to precipitate 141 g-eq of calcium chloride, or 70.5 mol of sodium carbonate is required for 70.5 mol of calcium chloride. Then,
70.5 * 106 = 7473 grams of Na2CO3
Where, 106 g/mol is the molar mass of Na2CO3
To precipitate 70 g-eq of magnesium chloride, 70 g-eq of sodium hydrate is required, or 35 mol of magnesium chloride requires 70 mol of sodium hydrate.
70 * 40 = 2800 grams of NaOH
Where, 40 g/mol is the molar mass of NaOH.
Taking into account the product of solubility, it is necessary to add technical and caustic soda with a slight excess.
Let us finally assume that for the complete precipitation of hardness salts, 7473 * 1.1 = 8220 grams of Na2CO3 and 2800 * 1.1 = 3080 grams of NaOH are required.
To transfer excess hydrate into water with the formation of sodium chloride, hydrochloric acid must be dosed into waste water after precipitation.
The consumption of hydrochloric acid will be equal to the excess amount of hydrate in the waste water. For this example, 1 mol of Na2CO3 will consume 0.5 mol of HCl and 1 mol of NaOH will consume 1 mol of HCl.
We get 7.05 * 36.5 + 7 * 36.5 = 513 grams of HCl.
Thus, the total cost of reagents for one regeneration will be:
Na2CO3 – 8.220 * 0.3 = 2.46 $.
Where, 0.3 $/kg – the average cost of 1 kg of technical and caustic soda in bulk purchases. 1.3 $/kg – the cost of 1 kg of hydrochloric acid.
NaOH – 2.800 * 0.3 = 0.84 $.
HCl – 0.5 * 1.3 = 0.65 $.
The cost of reagents for one regeneration: 2.46 + 0.84 + 0.65 = 3.95 $.
If we assume that there are two regenerations per day, then the total number of regenerations per year will be 660.
That, annual costs for reagents will be 3.95 * 660 = 2607 $/year.
Let’s calculate the annual cost of tableted table salt in case the regeneration was carried out with tableted salt.
The regeneration consumes 26.4 kg of tableted salt. With an average cost of 1 kg of salt equal to 0.3 $, we get 26.4 * 660* 0.3 = 5227.2 $/year.
In the case of wastewater treatment with soda, the savings compared to the option where tableted salt is used is half. At the same time, there are no highly mineralized effluents from the regeneration process of the softening unit.
Another important advantage of using soda is that no matter how much sodium is contained in the source water, a decrease in the filter cycle will not be required to compensate for its effect or increase the salt consumption for regeneration. Because the amount of hardness ions in any case will be removed from the water in a very close ratio to stoichiometric. At the same time, to ensure ion exchange, it is possible to maintain an arbitrarily much “inert” sodium chloride in the regeneration circuit. This circumstance allows you to save a significant amount of salt in the case of water purification with an initial high sodium content, which significantly reduces the exchange capacity of the cation exchanger and requires more salt for its regeneration.
Let’s return to the issue of concentration of treated wastewater up to 70-80 g/l. This salinity of the solution allows the regeneration of the cation exchanger.
After removal of hardness ions from waste water, the water has a concentration of about 16-20 g/l. It is necessary to concentrate sodium salts in this water. That is 4.5 – 5 times.
In principle, two methods can be used for these purposes. This is evaporation and reverse osmosis.
At first glance, water evaporation seems to be more economically preferable for the given task of obtaining a concentrated solution. But in practice, the equipment will take up a lot of space and require constant operation and maintenance. Also, the evaporating plant will require a coolant, which significantly complicates the concept and, accordingly, the automation of the process.
For this purpose, it is much more efficient, at least for small water softeners, to use reverse osmosis. In the process of reverse osmosis, a saturated concentrate and filtrate with low mineralization are formed. As a rule, it is the filtrate that is the main product of the reverse osmosis plant. But in this case, it is the concentrate that is the desired product, because with a fourfold concentration of the initial water with a mineralization of 20 g/l, it contains 80 g/l of sodium salts. And it is this concentrate that is used as a regeneration working solution of the softener cation exchanger. The resulting filtrate is returned to the purified water, because it is high quality purified water.
Structurally, a reverse osmosis plant is a conventional osmosis for seawater desalination. Due to the fact that it is necessary to obtain the concentrate with the salinity of about 80 g/l, the pressure for the reverse osmosis separation process will be about 80-82 bar, which is the maximum allowable pressure for SW type membranes. This circumstance must be taken into account when designing such installations.
The main disadvantage of using reverse osmosis for these purposes is the large consumption of electrical energy to ensure the process of reverse osmosis. Nevertheless, the equipment is compact, fully automated and does not require any other energy sources other than electrical energy.
The reverse osmosis-based wastewater disposal system is fully automated and does not require permanent maintenance personnel.
Conclusions:
- Utilization of waste water from the Na – cation exchange water softening plant by dilution does not make economic sense, because the amount of water consumed by the installation, in some cases, increases to 100%.
- Utilization of wastewater from a softener by precipitation of hardness salts using caustic and technical soda, followed by correction with hydrochloric acid, seems to be a highly efficient process, as a result of which reagents for the regeneration of the softener are significantly saved and there is no wastewater at all. As a result of the process, only sludge of a certain moisture condition is formed.
- The use of the technology of reverse osmosis water desalination in order to obtain a concentrate saturated with sodium salts as a regeneration working solution for a softening unit seems to be a rather effective technology. This process is simple, fully automated and does not require permanent maintenance personnel.

